Section: Opinion & Analysis
A Review of Deal Making in 2011
Pharmaceutical deal activity fell in 2011 as pharmaceutical companies cut R&D expenditure and streamlined their research activities. The need to partner remained, however, and the most sought after assets commanded sizeable premiums in 2011. Reflecting the difficult funding environment for biotech companies, M&A activity remained robust in 2011 and M&A deal values rose, although contingent payments were commonplace. Oncology continued to dominate the deal-making landscape and Roche, the leading player in this market, was the most prolific dealmaker.
After showing signs of recovery in 2010 following the global economic downturn of 2008/2009, the level of deal making in the pharmaceutical industry fell in 2011 as pharmaceutical companies tightened their belts and chose to rationalise their R&D activities. A review of the PharmaDeals® v4 database of publicly disclosed deal activity reveals that the number of deals signed in the pharmaceutical industry decreased by approximately 18% from 2010 to 2011 (Figure 1). Indeed, deal-making activity in 2011 dropped to the level seen in 2009. Partnering remains of huge strategic importance to pharmaceutical companies, however, as R&D productivity declines, blockbuster drug revenues fall off the patent cliff and growth in the major markets stalls.
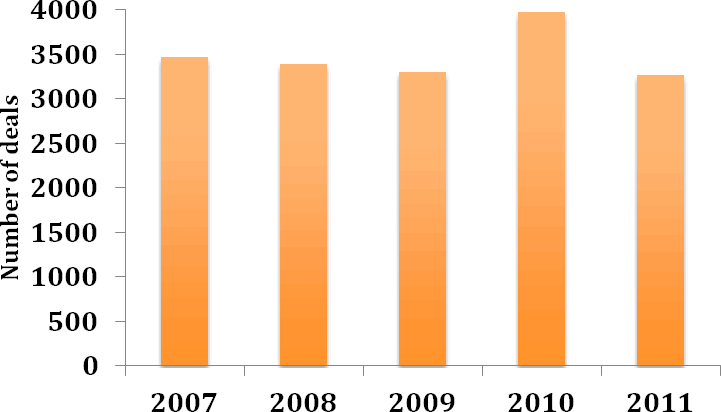 Figure 1: Number of deals 2007-2011
Figure 1: Number of deals 2007-2011
(Source: PharmaDeals® v4)
Collaborative R&D deals, which have declined in number in recent years, now appear to be plateauing, with a similar number of such deals being entered into in 2011 as in 2010 (Figure 2). The average total deal value, excluding royalties, of collaborative R&D deals with published financial figures rose slightly in 2011, halting the downward trend that has been observed since 2007 (Figure 3). An increase in the breadth of R&D collaborations, which often concern multiple targets and/or therapy areas, may in part explain this. A pertinent example is provided by the December 2011 collaboration between Abbott and Reata Pharmaceuticals for the development and commercialisation of Reata’s portfolio of preclinical, second-generation oral antioxidant inflammation modulators (Deal no. 44399). The collaboration covers a range of molecules across various therapeutic areas, including pulmonary, CNS disorders and immunology, and the US$400 M upfront payment ranks among the highest ever disclosed for a preclinical-stage deal.
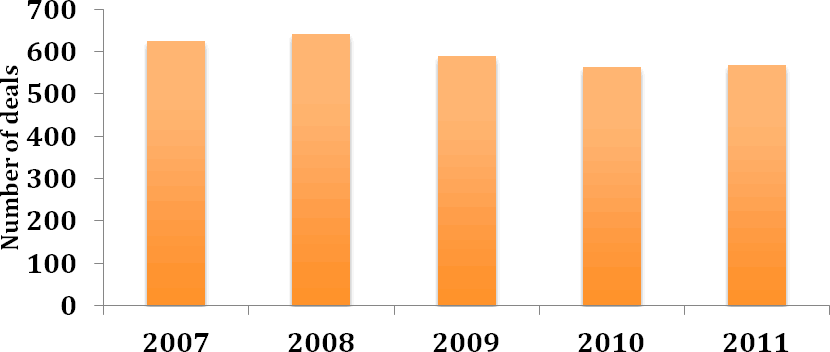 Figure 2: Number of collaborative R&D deals 2007-2011
Figure 2: Number of collaborative R&D deals 2007-2011
(Source: PharmaDeals® v4)
Options remain a tool frequently used by pharmaceutical companies in early-stage deals to mitigate risk. In June 2011, for example, Roche’s Genentech unit received exclusive global rights to acquire a preclinical-stage, small molecule cancer programme from Forma Therapeutics at a defined future phase of development (Deal no. 41557). The following month, Sanofi entered into a global research collaboration and licensing option with Rib-X Pharmaceuticals to develop and commercialise novel classes of antibiotics resulting from Rib-X’s RX-04 programme for the treatment of drug-resistant pathogens (Deal no. 41737).
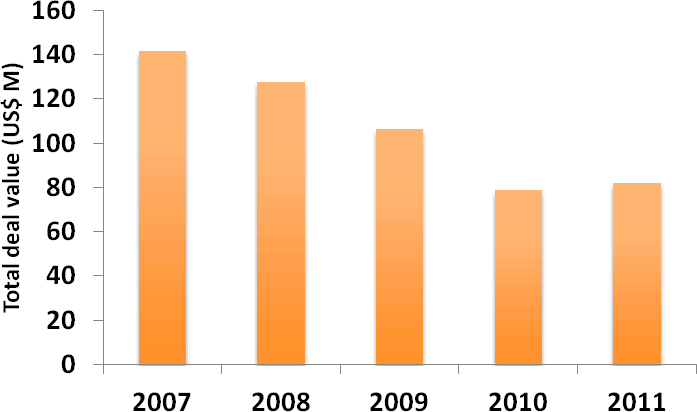 Figure 3: Average total deal value of collaborative R&D deals 2007-2011
Figure 3: Average total deal value of collaborative R&D deals 2007-2011
(Source: PharmaDeals® v4)
Owing to decreases in federal grant support towards scientific research and the decline in R&D productivity, collaborations between pharmaceutical companies and academic institutions are increasingly being pursued with the aim of commercialising therapeutic innovations. In August 2011, Pfizer formed a drug discovery and development partnership with University of California, San Diego, which is potentially worth up to US$50 M over 5 years, through its Centers for Therapeutic Innovation (CTI) (Deal no. 42376). Pfizer’s CTI, which exemplify a change in how big pharma interacts with academic research institutes, are a network of collaborative partnerships with life science research institutions that mimic venture capital-funded biotechs and which aim to bridge the gap between scientific discovery and the delivery of drug candidates into the clinical development pipeline.
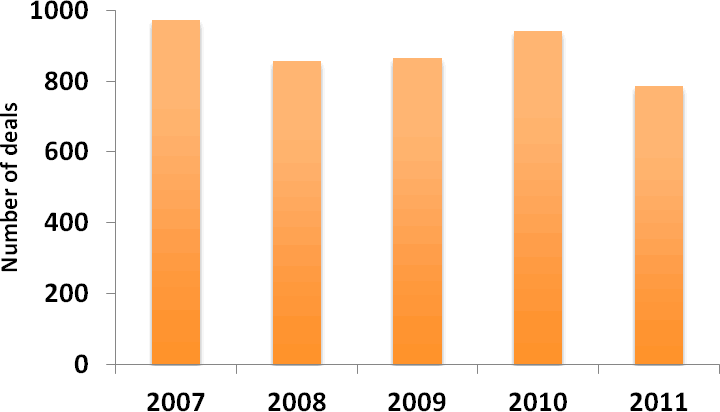 Figure 4: Number of licensing deals 2007-2011
Figure 4: Number of licensing deals 2007-2011
(Source: PharmaDeals® v4)
Licensing activity in the pharmaceutical industry, which has fluctuated in recent years, decreased by more than 16% from 2010 to 2011 (Figure 4). Moreover, the average total deal value, excluding royalties, of licensing deals with disclosed financial information fell further in 2011 from the high seen in 2009 (Figure 5). These trends may reflect an increasing discernment on the part of pharmaceutical companies that have been narrowing their therapeutic focus and reining in their expenditure. The observed fall in licensing deal values also suggests that licensees remain in a strong negotiating position owing to the limited financing options available to biotech companies, thanks to a weak IPO market and declining levels of venture capital funding.
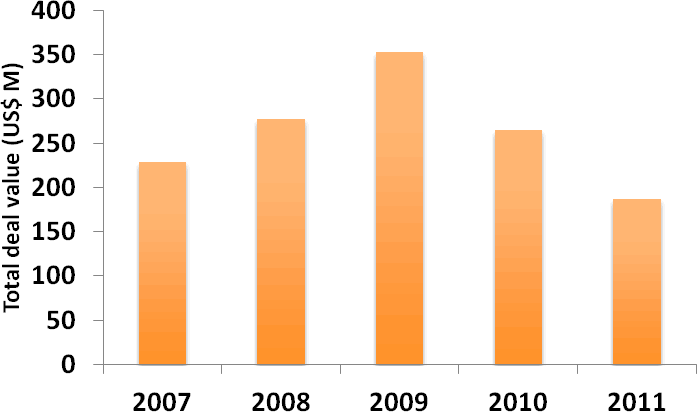 Figure 5: Average total deal value of licensing deals 2007-2011
Figure 5: Average total deal value of licensing deals 2007-2011
(Source: PharmaDeals® v4)
While the number of licensing deals for preclinical, Phase I and Phase III assets increased from 2010 to 2011, the number of Phase II licensing deals fell by 16% suggesting a shift to earlier stage partnering (Figure 6). Few of the Phase III licensing deals that were entered into in 2011 involved big pharma as licensees and those that did were typically regional licensing agreements such as Bayer Pharma’s deal for the development and commercialisation of Trius Therapeutics’ Phase III antibiotic, torezolid phosphate (Deal no. 42162).
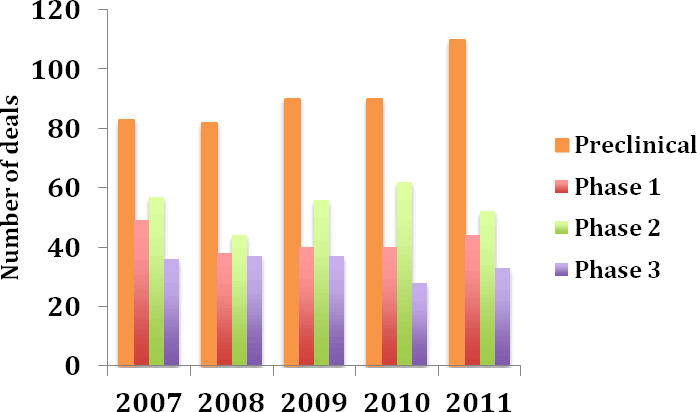 Figure 6: Number of licensing deals by development stage 2007-2011
Figure 6: Number of licensing deals by development stage 2007-2011
(Source: PharmaDeals® v4)
In spite of the overall decline in deal-making activity in 2011, the level of M&A activity (defined here as Mergers and Business Acquisitions) remained robust, with a similar number of transactions being entered into in 2011 as in 2010 (Figure 7). Again, this likely reflects the difficult funding environment for biotech companies. Interestingly, the average value of M&A transactions rose by more than 30% in 2011, as competition for the most desirable assets pushed up premiums (Figure 8). In today’s risk-averse deal-making climate, however, private biotech M&A transactions increasingly involve sizeable contingent payments. In March 2011, for example, Cephalon agreed to buy cancer drug developer Gemin X Pharmaceuticals for US$225 M upfront plus up to an additional US$300 M upon the achievement of certain regulatory and sales milestones (Deal no. 39893).
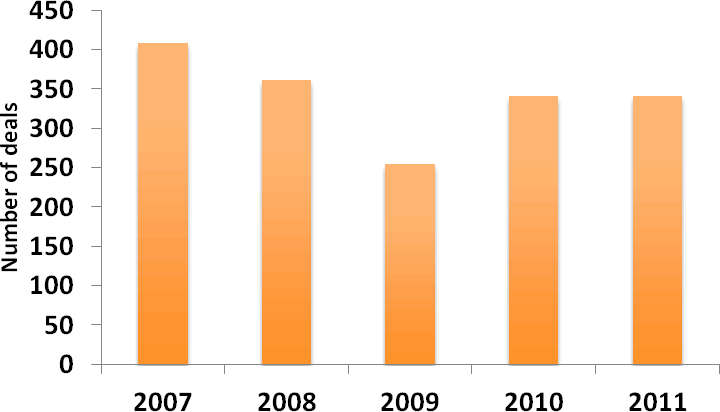 Figure 7: Number of M&A transactions 2007-2011
Figure 7: Number of M&A transactions 2007-2011
(Source: PharmaDeals® v4)
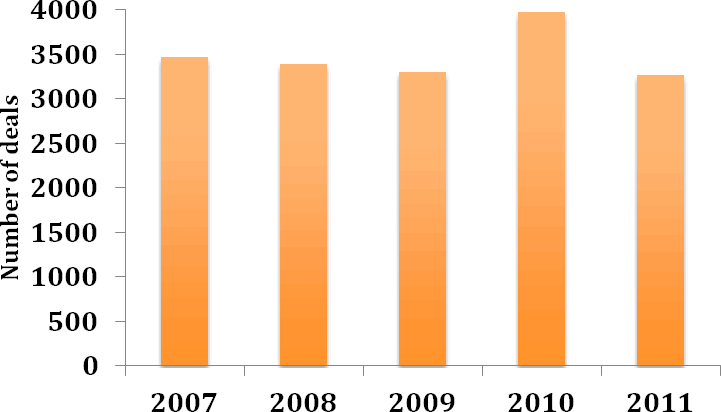 Figure 1: Number of deals 2007-2011
Figure 1: Number of deals 2007-2011(Source: PharmaDeals® v4)
Collaborative R&D deals, which have declined in number in recent years, now appear to be plateauing, with a similar number of such deals being entered into in 2011 as in 2010 (Figure 2). The average total deal value, excluding royalties, of collaborative R&D deals with published financial figures rose slightly in 2011, halting the downward trend that has been observed since 2007 (Figure 3). An increase in the breadth of R&D collaborations, which often concern multiple targets and/or therapy areas, may in part explain this. A pertinent example is provided by the December 2011 collaboration between Abbott and Reata Pharmaceuticals for the development and commercialisation of Reata’s portfolio of preclinical, second-generation oral antioxidant inflammation modulators (Deal no. 44399). The collaboration covers a range of molecules across various therapeutic areas, including pulmonary, CNS disorders and immunology, and the US$400 M upfront payment ranks among the highest ever disclosed for a preclinical-stage deal.
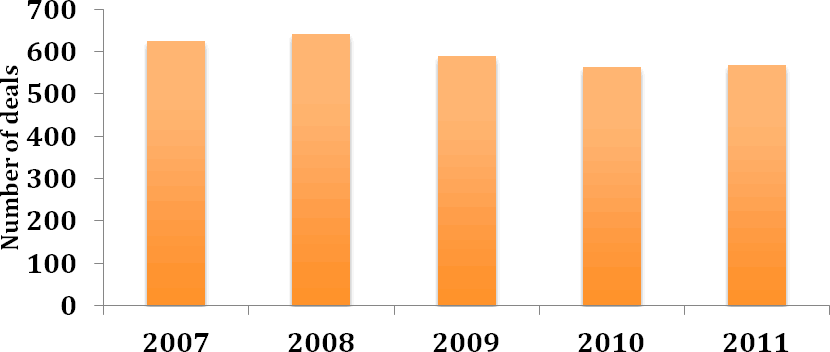 Figure 2: Number of collaborative R&D deals 2007-2011
Figure 2: Number of collaborative R&D deals 2007-2011(Source: PharmaDeals® v4)
Options remain a tool frequently used by pharmaceutical companies in early-stage deals to mitigate risk. In June 2011, for example, Roche’s Genentech unit received exclusive global rights to acquire a preclinical-stage, small molecule cancer programme from Forma Therapeutics at a defined future phase of development (Deal no. 41557). The following month, Sanofi entered into a global research collaboration and licensing option with Rib-X Pharmaceuticals to develop and commercialise novel classes of antibiotics resulting from Rib-X’s RX-04 programme for the treatment of drug-resistant pathogens (Deal no. 41737).
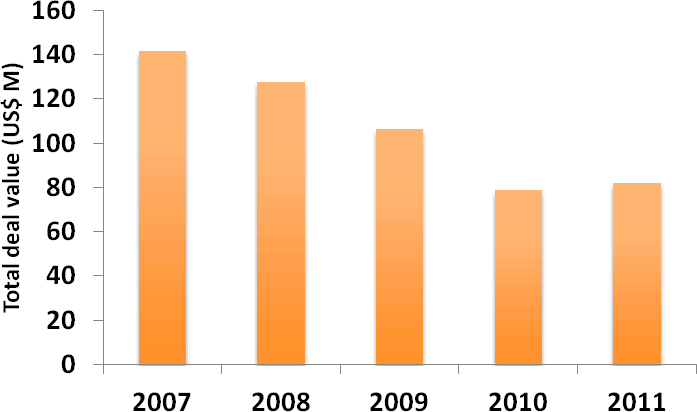 Figure 3: Average total deal value of collaborative R&D deals 2007-2011
Figure 3: Average total deal value of collaborative R&D deals 2007-2011(Source: PharmaDeals® v4)
Owing to decreases in federal grant support towards scientific research and the decline in R&D productivity, collaborations between pharmaceutical companies and academic institutions are increasingly being pursued with the aim of commercialising therapeutic innovations. In August 2011, Pfizer formed a drug discovery and development partnership with University of California, San Diego, which is potentially worth up to US$50 M over 5 years, through its Centers for Therapeutic Innovation (CTI) (Deal no. 42376). Pfizer’s CTI, which exemplify a change in how big pharma interacts with academic research institutes, are a network of collaborative partnerships with life science research institutions that mimic venture capital-funded biotechs and which aim to bridge the gap between scientific discovery and the delivery of drug candidates into the clinical development pipeline.
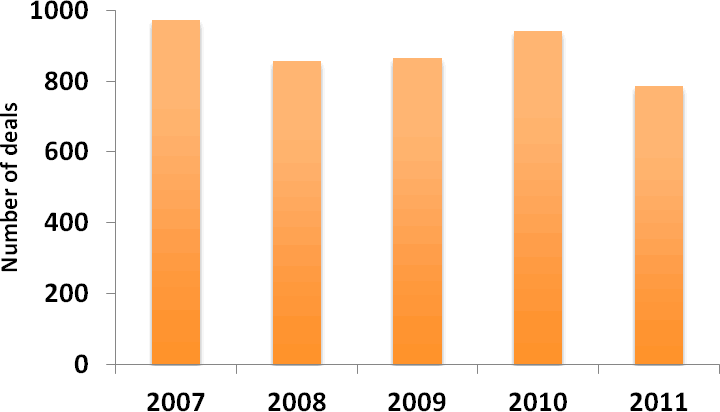 Figure 4: Number of licensing deals 2007-2011
Figure 4: Number of licensing deals 2007-2011(Source: PharmaDeals® v4)
Licensing activity in the pharmaceutical industry, which has fluctuated in recent years, decreased by more than 16% from 2010 to 2011 (Figure 4). Moreover, the average total deal value, excluding royalties, of licensing deals with disclosed financial information fell further in 2011 from the high seen in 2009 (Figure 5). These trends may reflect an increasing discernment on the part of pharmaceutical companies that have been narrowing their therapeutic focus and reining in their expenditure. The observed fall in licensing deal values also suggests that licensees remain in a strong negotiating position owing to the limited financing options available to biotech companies, thanks to a weak IPO market and declining levels of venture capital funding.
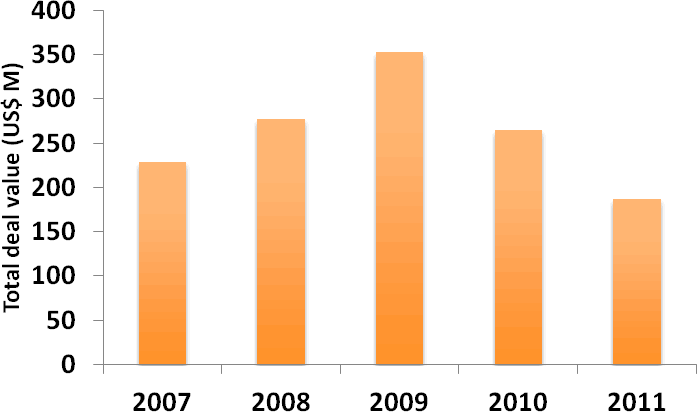 Figure 5: Average total deal value of licensing deals 2007-2011
Figure 5: Average total deal value of licensing deals 2007-2011(Source: PharmaDeals® v4)
While the number of licensing deals for preclinical, Phase I and Phase III assets increased from 2010 to 2011, the number of Phase II licensing deals fell by 16% suggesting a shift to earlier stage partnering (Figure 6). Few of the Phase III licensing deals that were entered into in 2011 involved big pharma as licensees and those that did were typically regional licensing agreements such as Bayer Pharma’s deal for the development and commercialisation of Trius Therapeutics’ Phase III antibiotic, torezolid phosphate (Deal no. 42162).
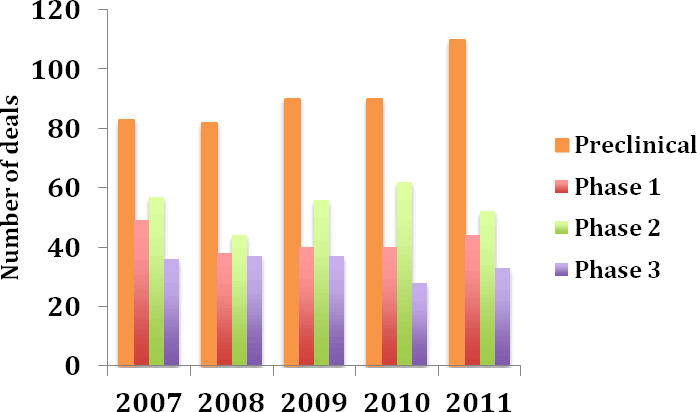 Figure 6: Number of licensing deals by development stage 2007-2011
Figure 6: Number of licensing deals by development stage 2007-2011(Source: PharmaDeals® v4)
In spite of the overall decline in deal-making activity in 2011, the level of M&A activity (defined here as Mergers and Business Acquisitions) remained robust, with a similar number of transactions being entered into in 2011 as in 2010 (Figure 7). Again, this likely reflects the difficult funding environment for biotech companies. Interestingly, the average value of M&A transactions rose by more than 30% in 2011, as competition for the most desirable assets pushed up premiums (Figure 8). In today’s risk-averse deal-making climate, however, private biotech M&A transactions increasingly involve sizeable contingent payments. In March 2011, for example, Cephalon agreed to buy cancer drug developer Gemin X Pharmaceuticals for US$225 M upfront plus up to an additional US$300 M upon the achievement of certain regulatory and sales milestones (Deal no. 39893).
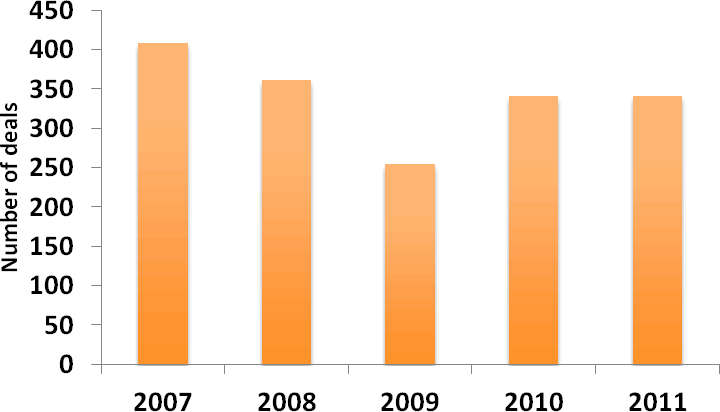 Figure 7: Number of M&A transactions 2007-2011
Figure 7: Number of M&A transactions 2007-2011(Source: PharmaDeals® v4)
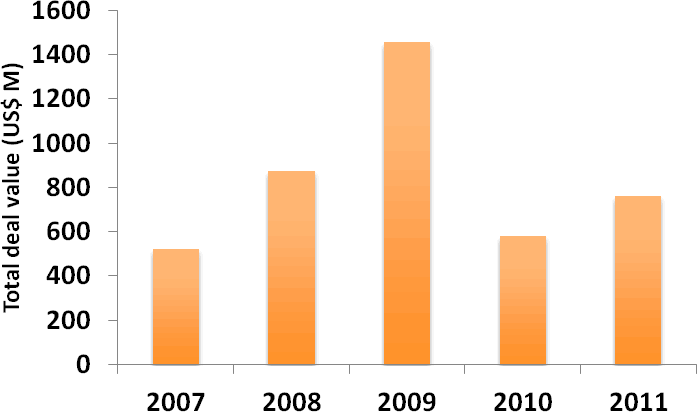 Figure 8: Average total deal value of M&A transactions 2007-2011
Figure 8: Average total deal value of M&A transactions 2007-2011(Source: PharmaDeals® v4)
By far the largest M&A deal of 2011 was Johnson & Johnson’s acquisition of the orthopaedic device company Synthes for US$21.3 B (Deal no. 40433). 2011 also saw a number of unprecedented M&A transactions. Takeda Pharmaceutical’s €9.6 B (US$13.7 B) purchase of Nycomed, a deal that broadened the company’s global footprint and boosted its position in the global pharmaceutical market, represents the largest takeover by a Japanese pharmaceutical company (Deal no. 40811). While Gilead Sciences’ US$11 B purchase of Pharmasset and its Phase III hepatitis C virus (HCV) nucleotide analogue is the largest ever acquisition of a clinical-stage biotech (Deal no. 44156).
Emerging markets remained at the top of the deal-making agenda for many of the major pharmaceutical companies in 2011. Faced with declining sales growth in Western markets, these companies are looking to exploit the substantial opportunities that emerging markets, and India and China in particular, provide. AstraZeneca, for example, moved to strengthen its position in China in December 2011 by agreeing to acquire Guangdong BeiKang Pharmaceutical, a manufacturer of generic injectable antibiotics (Deal no. 44383). Other big pharma companies went down the joint venture route in 2011 with Bayer HealthCare partnering with Zydus Cadila to establish a sales and marketing joint venture in India (Deal no. 39013) and Merck & Co. following suit by creating a large-scale joint venture with Sun Pharmaceutical Industries to develop and commercialise novel combinations and formulations of branded generics in emerging markets (Deal no. 40162).
Oncology continues to dominate the pharmaceutical deal-making landscape. As has been the case for the past 5 years, neoplasms remained the top therapy area for deal making in 2011 by some distance (Figure 9). This reflects the size of the market opportunity in oncology, particularly for first-in-class therapies with novel mechanisms of action, as well as a shift towards the development of personalised therapeutics. Indeed, pharmaceutical companies are increasingly linking up with diagnostics companies to develop companion diagnostics in order to identify which patients are likely to benefit most from treatment with their drug candidates. Novartis’ acquisition of the US diagnostic laboratory services company Genoptix was a notable example of this trend in 2011 (Deal no. 38966).
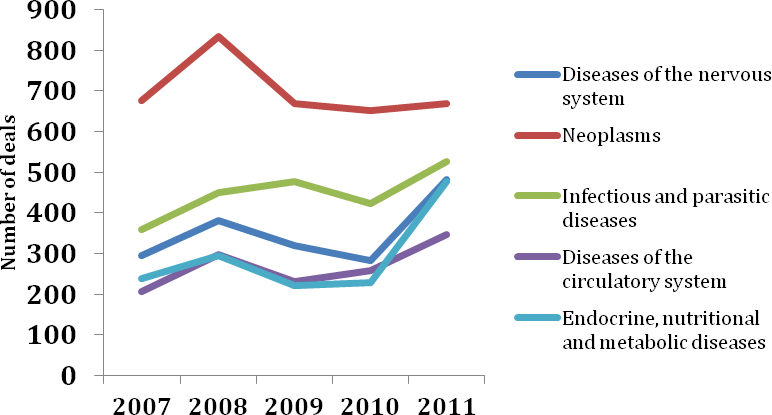 Figure 9: Number of deals by therapy area (selected therapy areas only)
Figure 9: Number of deals by therapy area (selected therapy areas only)(Source: PharmaDeals® v4)
Infectious and parasitic diseases comprised the second most popular therapy area for deal making in 2011, although the number of deals in this category was down 20% on 2010. Fuelled by the approvals of Vertex Pharmaceuticals’ Incivek™ (telaprevir) and Merck & Co.’s Victrelis™ (boceprevir), the first novel HCV drugs to gain regulatory approval in almost a decade, the rapidly evolving HCV market was the subject of a number of noteworthy deals in 2011, including Gilead’s acquisition of Pharmasset and Vertex’s US$1.5 B global licensing agreement with Alios BioPharma for two preclinical-stage nucleotide analogues (Deal no. 41275). Diseases of the nervous system, endocrine, nutritional and metabolic diseases and diseases of the circulatory system comprised the third, fourth and fifth most popular therapy areas for deals signed in 2011, respectively.
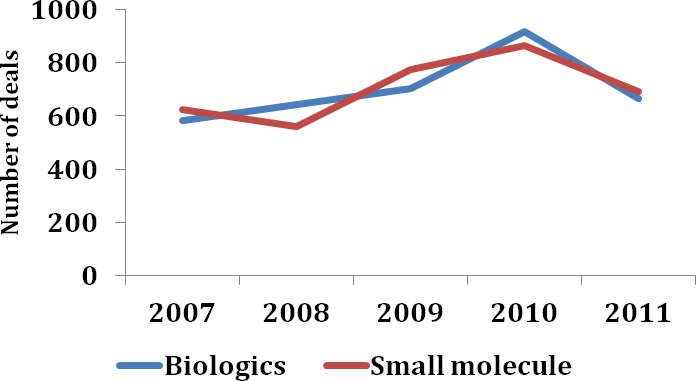 Figure 10: Number of therapeutic deals for small molecules and biologics
Figure 10: Number of therapeutic deals for small molecules and biologics(Source: PharmaDeals® v4)
After several years of growth, biologics deals declined significantly in number in 2011, falling behind deals for small molecule therapeutics (Figure 10). This may in part be explained by the nature of the biologics deals that were signed in 2011, many of which involved broad access to platform technologies. Protein engineering particularly caught the attention of big pharma in 2011. Spurred by the development success of Adcetris™ (brentuximab vedotin), which gained US FDA approval for the treatment of Hodgkin’s lymphoma and systemic anaplastic large-cell lymphoma in August 2011, Seattle Genetics added a number of companies to its roster of collaborators for its antibody-drug conjugate technology in 2011, including Oxford BioTherapeutics (Deal no. 42989), Genmab (Deal no. 40317), Abbott (Deal no. 39859) and Pfizer (Deal no. 38736). Bispecific antibody platforms were also of interest. In August 2011, for example, Zymeworks partnered with Merck & Co. for the development of novel bispecific antibodies generated through use of its Azymetric™ platform (Deal no. 42743) while the following month F-star collaborated with Merck Serono to discover novel antibody-derived therapeutics against inflammatory disease targets using its modular antibody technology (Deal no. 42888). The latter part of the year saw a raft of deals for the development and commercialisation of biosimilars with companies such as Biogen Idec (Deal no. 44344), Amgen (Deal no. 44515) and Baxter International (Deal no. 44559) making entries into this market via deal making.
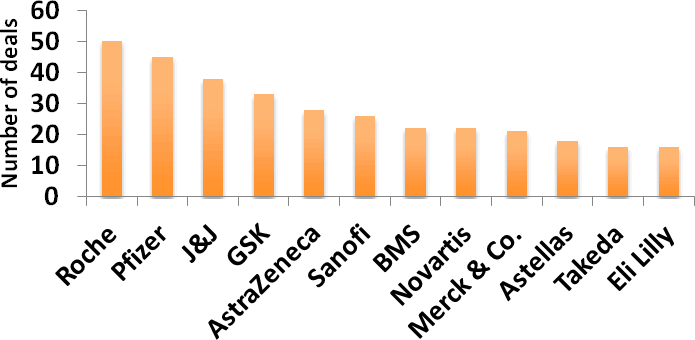 Figure 11: Top dealmakers of 2011
Figure 11: Top dealmakers of 2011(Source: PharmaDeals® v4)
Roche was the most prolific dealmaker of 2011, entering into more than twice the number of deals of some of its big pharma contemporaries (Figure 11). The company also ended the year leading the PharmaDeals Corp-METRx Deal Activity League Table, which ranks the top 12 pharmaceutical companies by their licensing activity over a 5-year period. Roche’s largest deal of 2011, by headline value, was a global licensing agreement for the development and commercialisation of Evotec’s monoamine oxidase type B (MAO-B) inhibitor EVT-302, which could slow the progression of Alzheimer’s disease and for which Roche plans to initiate a 12-month treatment Phase IIb study in 2012 (Deal no. 42885). Most of the total deal value is tied up in commercialisation milestones, however, and the upfront payment was only US$10 M. The company’s other deals were a mixed bag, reflecting the nature of its business operations, with diagnostics, hepatitis C and oncology featuring prominently.
In summary, a review of deal making in 2011 suggests that pharmaceutical companies are becoming increasingly selective in terms of the assets they choose to license or acquire in light of budget constraints and pipeline rationalisation. To gain the most sought after products in high-growth therapy areas, big pharma is being forced to pay substantial premiums as its own R&D engine stalls. The challenging funding environment for biotechs has helped drive a shift to earlier stage partnering, although these companies are often required to retain risk via option-based deal structures. The most active areas of deal making in 2011 included therapy areas with high unmet clinical need and technology platforms for the development of next-generation protein therapeutics.
